
WebQuelle est la mi-temps d'une réaction de premier ordre ? Réponse : La demi-vie de toute réaction est le temps pendant lequel la réaction est terminée à 50 %. La formule de la. WebFirst Order Reaction - Key takeaways. A first-order reaction is a reaction where the rate is dependent on the concentration of only one reactant. Because of this, it is also called an.
Show That In A First Order Reaction, t99.9%=10×t1/2 in a FIRST ORDER REACTION👍, 6.23 MB, 04:32, 13,103, ASN CHEMISTRY, 2019-11-07T17:30:27.000000Z, 19, Image result for first order reactions | Math worksheets, www.pinterest.com, 960 x 720, jpeg, order reactions calculus result google maths science, 20, show-that-in-a-first-order-reaction, KAMPION
WebThe term 'reaction order' (or order of reaction) refers to how the concentration of one or more reactants (chemicals) affects the rate of the reaction. The overall order. WebIn first-order reactions in which the rate-limiting step is the protonation of the reactant, the sorption of the reactant dominates the rates. Identical intrinsic rates of reactions were. WebFor a first order reaction, time required for 99.0 % completion is x times for the first time required for the completion of 90 % of the reaction x is: Q. For a first-order reaction, the. WebFor first order reaction, k = 2.303 t log ([A] 0 [A]) t 1 / 2 = 0.693 k For t 99.9 %; t 99.9 % = 2.303 k log (100 0.1) ⇒ t 99.9 % = 2.303 k log 1000 t 99.9 % = 2.303 k × 3 ⇒ t 99.9 % =. WebA first-order reaction is a chemical reaction in which the rate of the reaction is directly proportional to the concentration of the reactants. Put another way, the rate of a. WebA second order reaction can be altered to a first order reaction by taking one of the reactant in large excess, such reaction is called pseudo first order reaction. Let us. WebA first-order reaction is a chemical reaction where the reaction rate depends linearly on the reactant’s concentration. In other words, if the concentration is doubled, the reaction rate. WebIn case of a first-order reaction, the ratio of time required for 99.9% completion to 50% completion is: For a first-order reaction, the time required for 99.0% completion is. WebThe concentration v/s time graph for a first-order reaction is provided below. For first-order reactions, the equation ln [A] = -kt + ln [A] 0 is similar to that of a straight line (y = mx + c).
Discussion t99.9%=10×t1/2 in a FIRST ORDER REACTION👍 update
Show that in 1st order reaction time required for completion of 99.9% is 10 times of half life of it going viral
Other descriptions of Show That In A First Order Reaction updated for you
News Image result for first order reactions | Math worksheets

Articles Solved: Consider The First-order Reaction Described By The... | Chegg.com New
Discussion For a first order reaction - YouTube

First order reactions | Science, Chemistry, Physical Chemistry | ShowMe trending
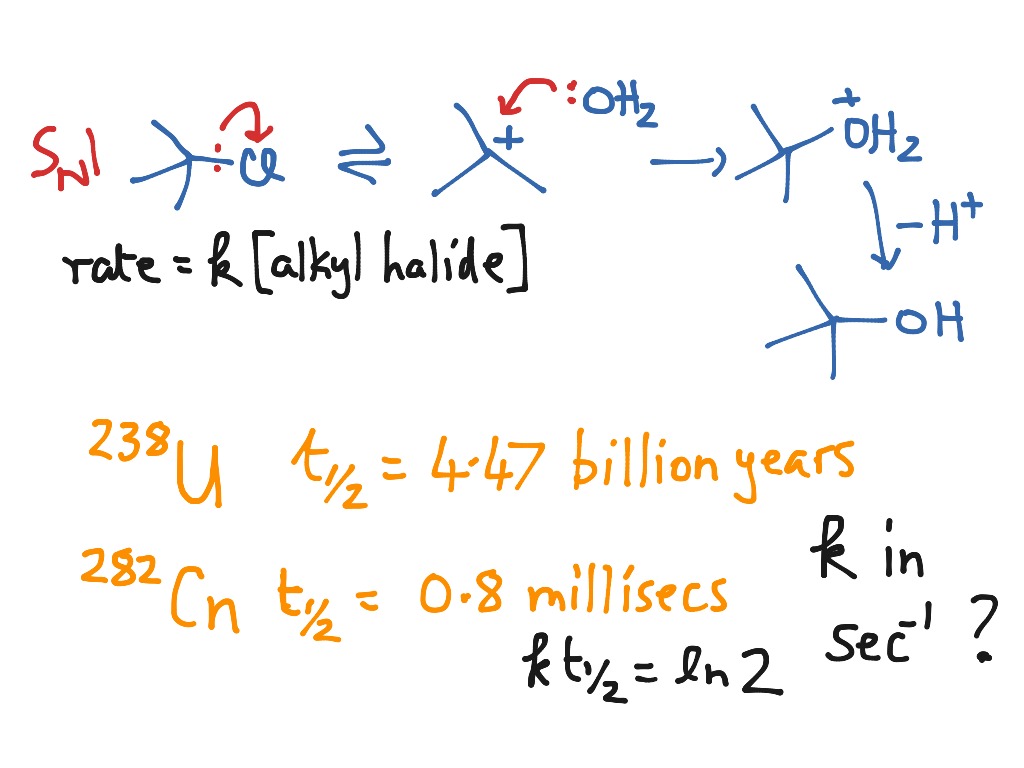
New PPT - Chemical Kinetics PowerPoint Presentation, free download - ID:5829521 Latest
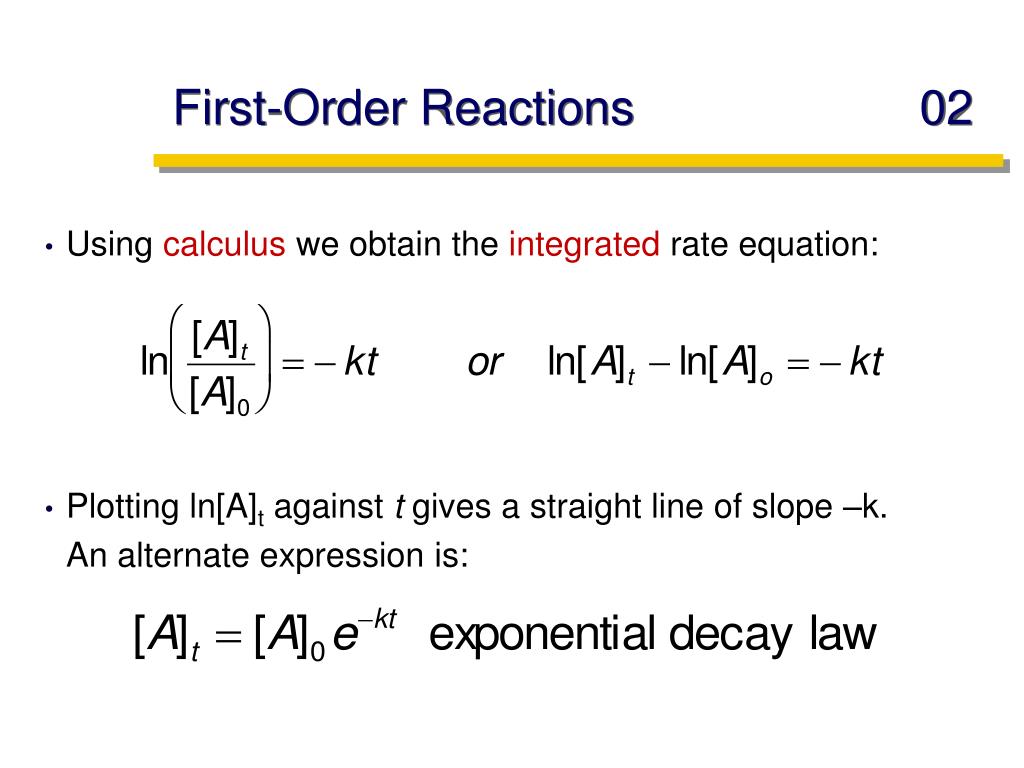
Watch PPT - Chemical kinetics or dynamics PowerPoint Presentation, free

News First order reaction example 2 - YouTube update

News First order complex reaction updated
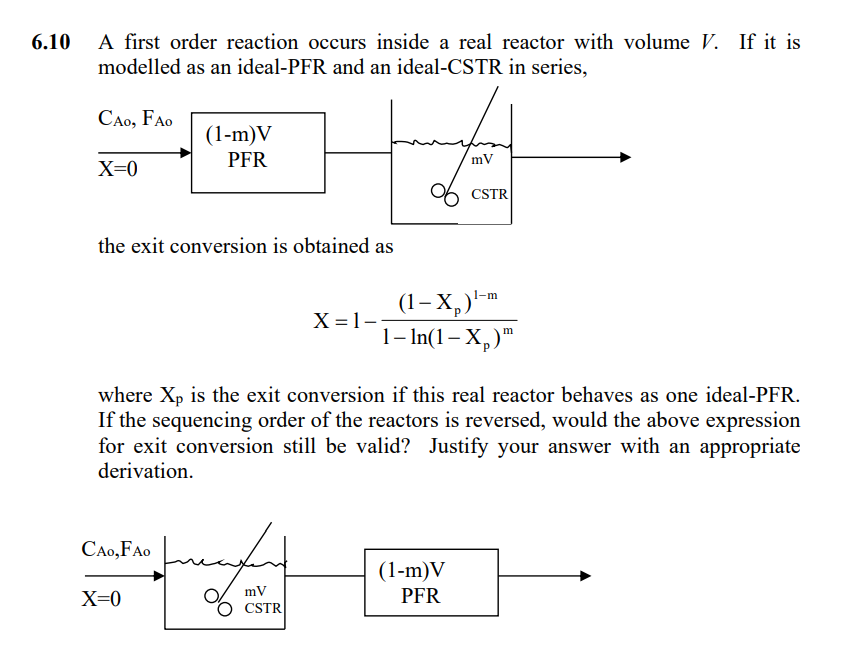
View Solved A first order reaction occurs inside a real reactor | Chegg.com Latest
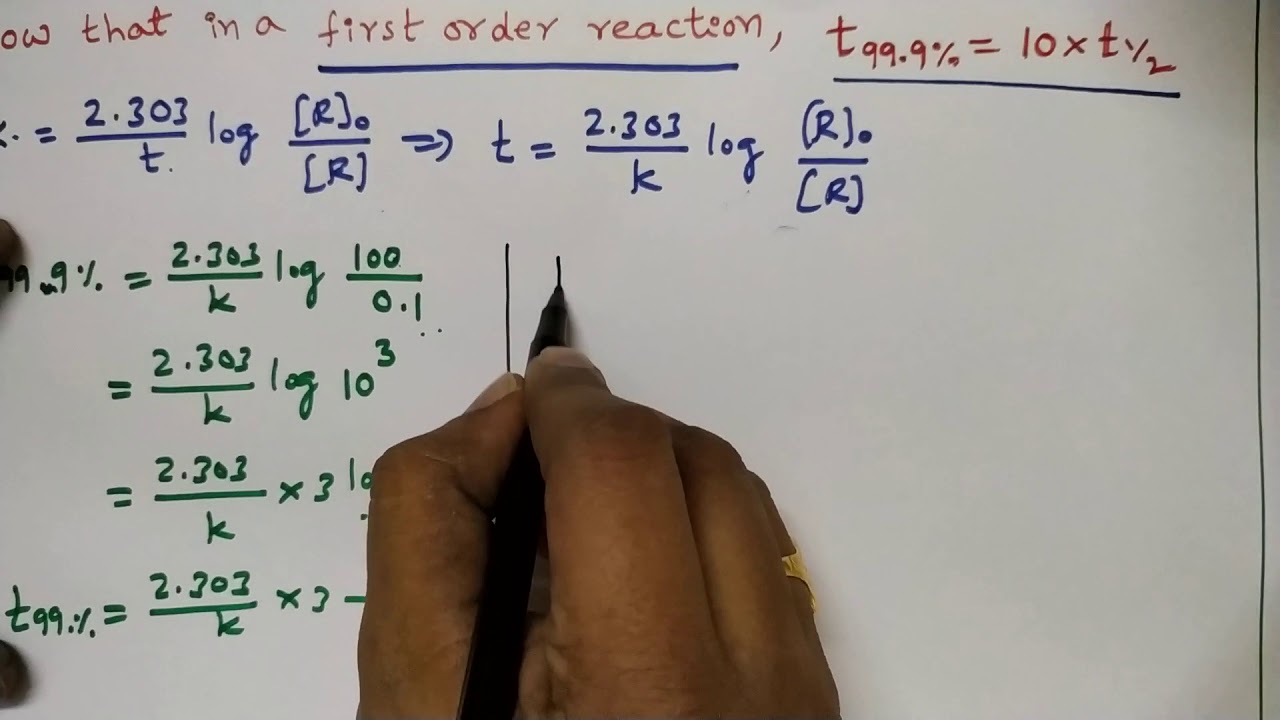

Belum ada tanggapan untuk "Let'S See Show That In A First Order Reaction Update"
Posting Komentar