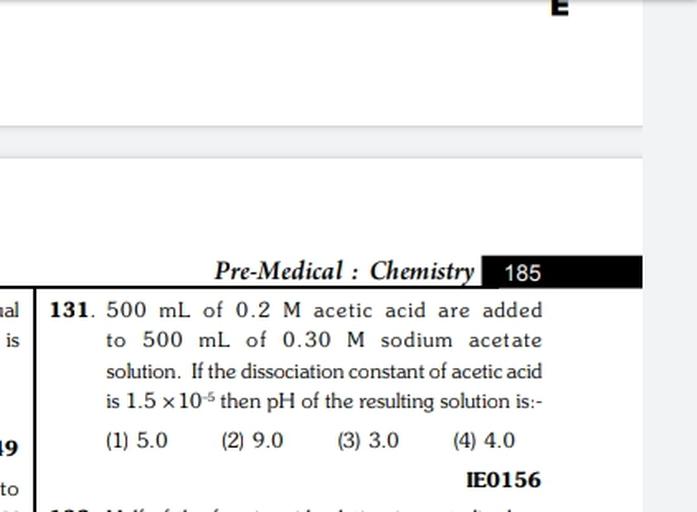
Web500 mL of 0.2 M acetic acid is added to 500 mL of 0.30 M sodium acetate solution. If the dissociation constant of acetic acid is 1.5×10 −5, then the pH of the resulting solution is: A. WebSimilarly, if i want to prepare 500 mL of 0.2% acetic acid, I would dissolve 1 mL of glacial acetic acid into 499 mL of water. Cite. 6 Recommendations. 28th Oct, 2020. Muhammad. Web500 mL of 0.2 M aqueous solution of acetic acid is mixed with 500 mL of 0.2 M HCl at 25∘ C 25 ∘ C . (i) Calculate the degree of dissociation of acetic acid in the.
500ml Of 0 2 M Acetic Acid, Calculate the pH of 0.1 M Acetic Acid, 9.73 MB, 07:05, 66,881, chemistNATE, 2019-12-01T16:34:10.000000Z, 19, al is Pre-Medical : Chemistry 185 131. 50... - Physical Chemistry, questions-in.kunduz.com, 697 x 512, jpeg, , 20, 500ml-of-0-2-m-acetic-acid, KAMPION
WebCalculate the volume of 0.500 M acetic acid and the volume of 0.500 M sodium acetate required to make 300.0 mL of a buffer solution with pH = 5.30. (The Ka. WebGlacial acetic acid, a highly concentrated solution of acetic acid, is available from several commercial suppliers and contains ≈99.7% (w/w) acetic acid. The molarity of this. Web`500mL` of `0.2M` aqueous solution of acetic acid is mixed with `500mL` of `0.2HCI` at `25^(@)C`. a. Calculate the degree of dissociation of acetic acid in the. WebThe given solution of acetic acid and sodium acetate is an acidic buffer solution as acetic acid is a weak acid while sodium acetate is the salt of weak acid acetic. WebQuestion From – KS Verma Physical Chemistry Class 11 Chapter 08 Question – 274 IONIC EQUILIBRIUM CBSE, RBSE, UP, MP, BIHAR BOARDQUESTION TEXT:-When `0.2M`. WebA solution is made by adding 50.0 mL of 0.200 M acetic acid (K 2 L8 * I0 to S0.0 IL of L.O * 10 MHICL 4. Calculate the pH of the solution. b Calculate the acetale iOn concentration.. WebQuestion From – KS Verma Physical Chemistry Class 11 Chapter 08 Question – 086 IONIC EQUILIBRIUM CBSE, RBSE, UP, MP, BIHAR BOARDQUESTION TEXT:-`500mL` of `0.... Web500mL of 0.2M acetic acid are added to 500mL of 0.30M sodium acetate solution. If the dissociation constant of acetic acid is 1.5 × 10^-5 then pH of the resulting solution is:. WebTo determine the pH of any solution we need to know the hydrogen ion concentration in moles per liter. In this case we have a 0.0500 M solution of acetic acid. That’s 0.05.
Watch Calculate the pH of 0.1 M Acetic Acid
View 0.1 molar acetic acid | acetic acid 0.1 molar | 0.1 m acetic acid
Explanation 500ml Of 0 2 M Acetic Acid in full
* Use Ka and the initial concentration to calculate the new concentration of H+ ions... you might need an ICE Table.
* Take the negative log of the H+ concentration to find pH.
Check me out: chemistnate.com
Images al is Pre-Medical : Chemistry 185 131. 50... - Physical Chemistry more

Look Acetic Acid, Reagent, 500 mL trending
Reviews Acetic Acid LR 500 ML, For Industrial, Packaging Type: Bottle, | ID New

ACETIC ACID GLACIAL 99.5% Extra Pure - Oxford Lab Fine Chem LLP updated
ACETIC ACID, glacial, UNILAB (500 ML) - Produsen Furniture Laboratorium popular

Currently - Acetic Acid, Reagent, 500 mL | Flinn Scientific update

A12800-500.0 - Acetic Acid, Glacial, 500 Milliliters popular

New Acetic Acid, 17.4 M (100% v/v), Glacial, ACS Grade, 500 mL | Carolina.com

Currently - Acetic Acid in Indore, सिरका अम्ल, इंदौर, Madhya Pradesh | Acetic Acid update

Reviews MERCK 159166 Acetic Acid 30% for analysis Reag. Ph Eur 500 mL New



Belum ada tanggapan untuk "Let'S See 500ml Of 0 2 M Acetic Acid Latest"
Posting Komentar