
WebA current of 2 amp when passed for 5 hour through a molten salt deposits 22.2 g of metal of atomic mass 177. Then calculate the positive oxidation state of the metal in the. WebHow long must a constant current of 50.0 A be passed through an electrolytic cell containing aqueous Cu2+ ions to produce 5.00 moles of copper metal? 0.373 hours 5.36 hours 2.68.
A Current Of 2 0 A Is Passed For 5 Hours, A current of \( 2.0 \) A passed for 5 hours through a molten metal ..., 5.68 MB, 04:08, 10, PW Solutions, 2022-08-08T06:12:02.000000Z, 19, A current of 2.0 ampere is passed for 5.0 hour through a molten tin, www.doubtnut.com, 1200 x 677, png, , 20, a-current-of-2-0-a-is-passed-for-5-hours, KAMPION
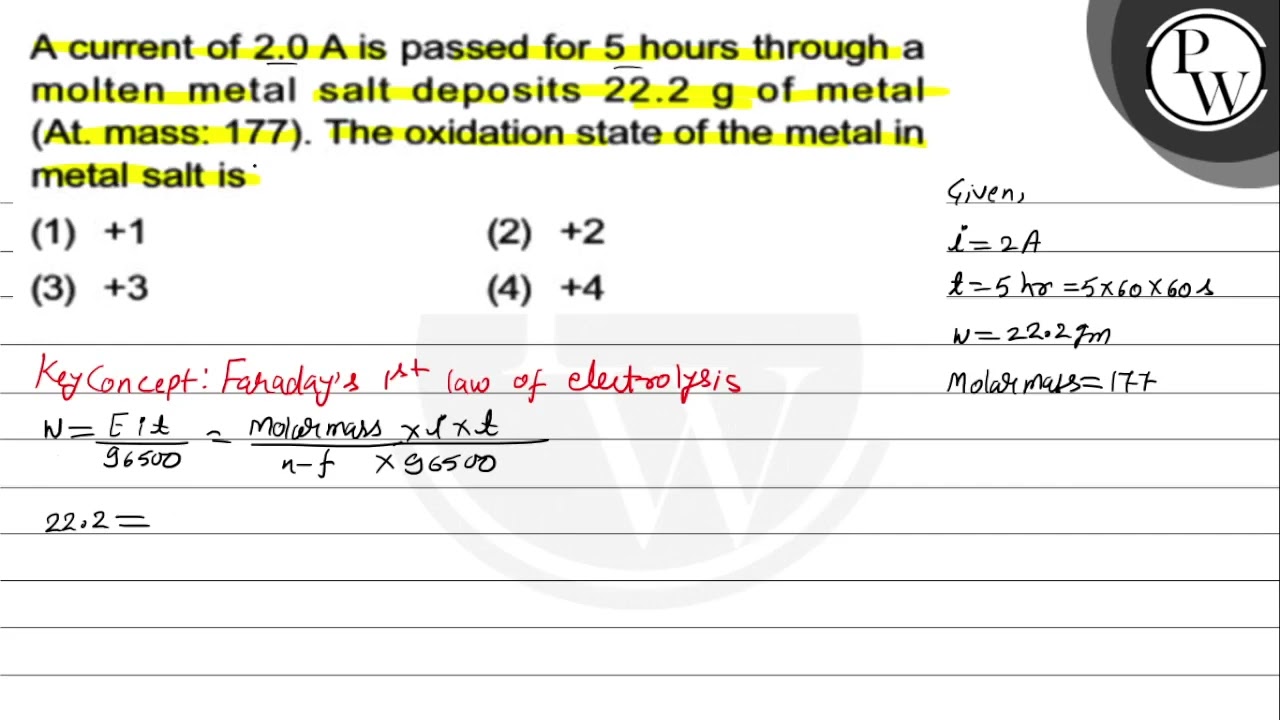
WebA current of 2.0 A passed for 5 hours through a molten metal salt deposits 22.2 g of metal (At. wt. = 177). The oxidation state of the metal in the metal salt is: A current of 2.0 A. WebExams. Chemistry. Electrolysis. A current of 2.0A passed for 5 hours through a molten metal ... Question: A current of 2.0\, A 2.0A passed for 5 5 hours through a molten. WebIn an electrolysis experiment, a current was passed for 5 hours through two cells connected in series. The first cell contains a solution of gold salt and the second cell. WebSolution. I = 0.5 A. t = 2 hours = 2 × 60 × 60 s = 7200 s. Thus, Q = It. = 0.5 A × 7200 s. = 3600 C. We know that number of electrons. Then, Hence, number of electrons will flow. WebA current of 1.2A was passed through an electrolytic cell containing dilute Tetraoxosulphate(VI) for 40minutes. Calculate the volume of gas produced at s.t.p. How. WebA current of 2.0 A is passed for 5 hours through a molten metal salt deposits 22.2 g of metal (At. mass: 177). The oxidation state of the metal in metal salt is The. WebVIDEO ANSWER: everyone in this question, they ask if the constant current is passed through a cell containing cr three plus for 2.5 hours, how many grams of CIA will Blacked. WebCurrent (I)= 50A. Quantity of electricity (Q) is given as, The half cell of the reaction can be shown as, 1 mole of copper is deposited by, If, 1 mole of Copper =.
A current of \( 2.0 \) A passed for 5 hours through a molten metal ...
Topics A current of \( 2.0 \mathrm{~A} \) is passed for 5 hours through a ... Latest
A Current Of 2 0 A Is Passed For 5 Hours latest
A current of \( 2.0 \) A passed for 5 hours through a molten metal salt deposits \( 22.2 \) \( \mathrm{g} \) of metal (At wt. = 177). The oxidation state of the metal in the metal salt is
(A) \( +1 \)
(B) \( +2 \)
(C) \( +3 \)
(D) \( +4 \)
📲PW App Link - bit.ly/YTAI_PWAP
🌐PW Website - pw.live
Watch A current of 2.0 ampere is passed for 5.0 hour through a molten tin Latest

Currently - A current of 2.0 A is passed for 5 hours through a molten metal salt going viral

About A current of `2.0` ampere is passed for `5.0` hour through a molten tin more

Discussion An electric current of 100 ampere is passed through a molten liquid of more

Watch A lamp draws a current of 2.0 A. Find the charge in coulombs used by updated

Photos A current of 5 13 A is passed through a Fe(NOs)z solution. How long (in viral

About During electrolysis of aqueous copper (II) sulphate using copper more

Photos 3. Find out the oxidation state of tin in its salt when 11gram of tin more
A current of 5 13 A is passed through a Fe(NOs)z solution. How long (in going viral

Let's see A current of 3 amperes is passed for 5 hours through a molten metal updated


Belum ada tanggapan untuk "View A Current Of 2 0 A Is Passed For 5 Hours Viral"
Posting Komentar