
WebThe molar conductivity of 0.025 mol L^–1 methanoic acid is 46.1 S cm^–1 mol^–1. Its degree of dissociation (α) and dissociation constant. Given λ^° (H^+) = 349.6. WebQ. Molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1, the degree of dissociation and dissociation constant will be : (Given: λo H+ =349.6 S cm2 mol−1 and λo.
The Molar Conductivity Of 0 025, The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calculate its degree...., 9 MB, 06:33, 4,933, Dr. Bandhana Sharma, 2021-09-02T16:51:17.000000Z, 19, The molar conductivity of 0.025 mol L^(-1) methanoic acid is 46.1 S cm, doubtnut.com, 1200 x 677, png, , 20, the-molar-conductivity-of-0-025, KAMPION
WebThe molar conductivity of 0.025 mol L 1 methanoic acid is 46.1 S cm 2 mol 1Calculate its degree of dissociation and dissociation constant. Given λ∘ H +=349.6 S cm 2 mol 1 and. WebThe molar conductivity of `0.025 mol L^(-1)` methanoic acid is `46.1 S cm^(2) mol^(-1)`. Its degree of dissociation `(alpha)` and dissociation constant. WebQuestion 3.9:The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given λ0(H+)= 349.6 S. WebThe molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol −1. Calculate its degree of dissociation and dissociation constant. Given λ ° (H +) = 349.6 S cm 2 mol. WebConcentration ,C = 0.025 mol L-1. λ(HCOOH) = 46.1 S cm2 mol−1. use formula. λ°(HCOOH) = λ0(H+) + λ0(HCOO–) plug the values we get. λ°(HCOOH) =. WebResistance of Conductivity cell when filled with a solution of electrolyte of concentrated 0.1 M is 100 ohms and conductivity of this solution is 1.2sm −1 when some cell is filled with. WebThe molar conductivity of 0.025 mol L-1 methanoic acid is. 46.1 S cm2 mol-1. Calculate its degree of dissociation and dissociation constant. Given λ 0 (H+) = 349.6 S cm 2 mol. WebMolar conductivity of 0.025 mol L-1 methanoic acid is 46.1 Scm 2 mol -1 the dissociation constant will be: (Given: λ H +0=349.6 S cm 2 mol -1 and λ WebClick here👆to get an answer to your question ️ Molar conductivity of 0.025 mol L^-1 methanoic acid is 46.1 S cm^2 mol^-1 , the degree of dissociation and dissociation.
The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calculate its degree.... going viral
Discussion The molar conductivity of `0.025 mol L^(-1)` methanoic acid is `46 popular
Read more from The Molar Conductivity Of 0 025 Next
NCERT Intext Question Page No. 85 ELECTROCHEMISTRY
Problem 3.9:- The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calculate its degree of dissociation and dissociation constant. Given λ0 (H+) = 349.6 S cm2 mol-1 and λ0 (HCOO-) = 54.6 S cm2 mol-1.
About The molar conductivity of 0.025 mol L^(-1) methanoic acid is 46.1 S cm Latest

Currently - The molar conductivity of 0.025 mol L^(-1) methanoic acid is 46.1 S cm more

Look The molar conductivity of 0.025 mol L^(-1) methanoic acid is 46.1 S cm New

Latest The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm^(-1 New

Articles Molar conductivity of CsBr in aqueous butan-2-ol mixture with w B

Please explain why limiting molar Conductivity of OH- is maximum viral

Topics The molar conductivity of 0.025 mol L^-1 methanoic acid is 46.1 S cm^2 trending

Photos Describe variation of molar conductivity with concentration of trending

Subject 25. The molar conductivity of HCI, NaOH a... - Physical Chemistry more
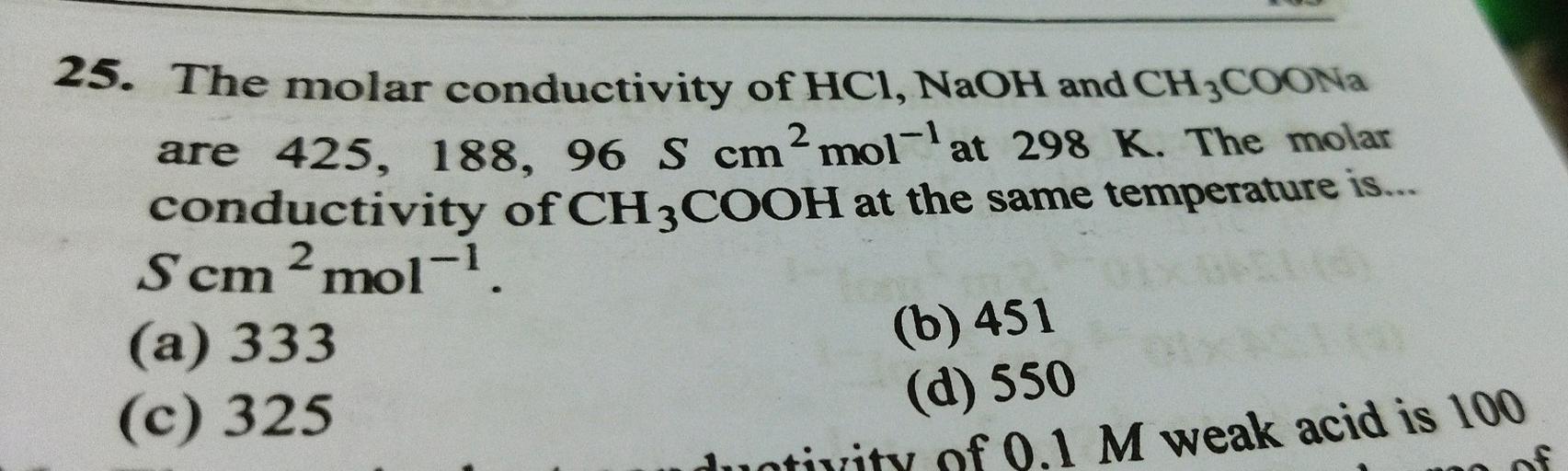


Belum ada tanggapan untuk "New The Molar Conductivity Of 0 025 For You"
Posting Komentar