
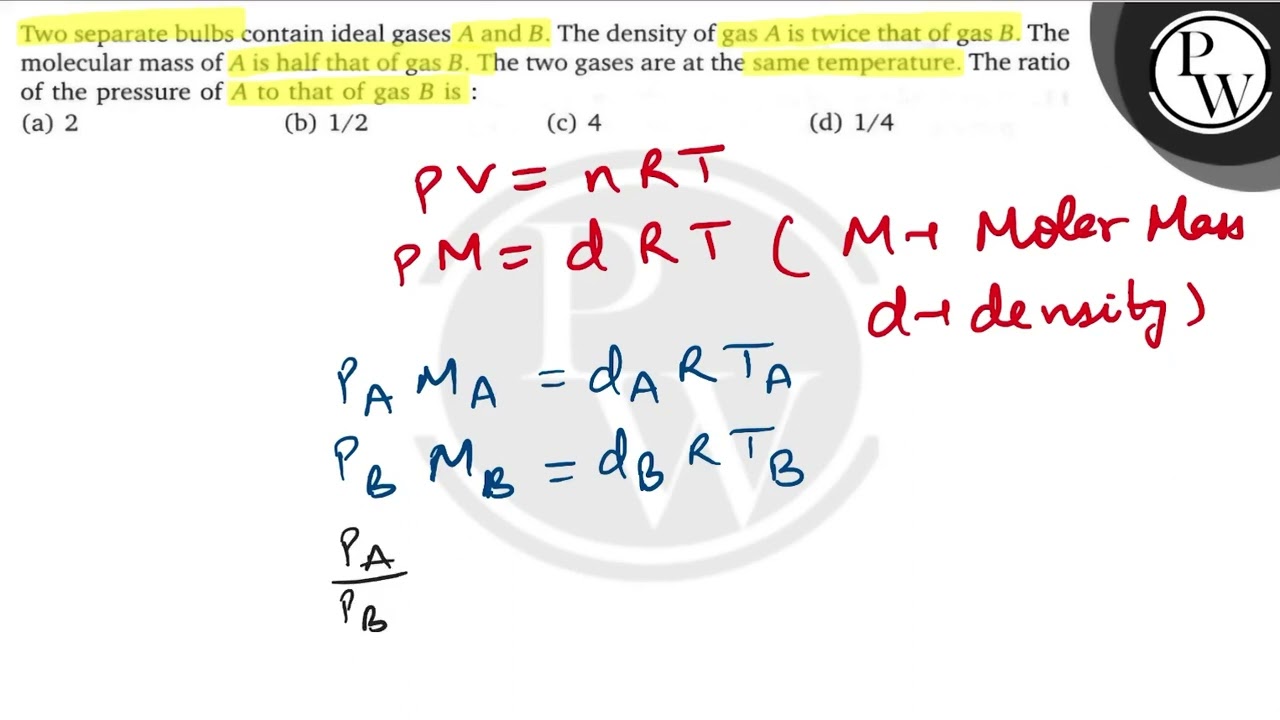
WebTwo separate bulbs contain ideal gases A and B. The density of A is twice as that of gas B. The molecular mass of gas A is half as that of B. If two gases are at same. WebTwo separate bulbs contain ideal gases A and B at the same temperature. The density of gas B is half of A while molar mass of gas B is twice of gas A. The ratio of pressure of. WebThe molecular mass of A is half that of B. The two gases are at the same temperature. The ratio of pressure A to that of gas B is? - Super Pathshala | SSC Railway Banking. Q. Two.
Two Separate Bulbs Contain Ideal Gases A And B, Two separate bulbs contain ideal gas `A` and `B`. The density of a gas `A` is twice that of a, 4.3 MB, 03:08, 378, Doubtnut, 2020-01-19T07:38:05.000000Z, 19, The vapour density of a aseous mixture of non-reactive gases 'A' and 'B, www.doubtnut.com, 1200 x 677, png, , 20, two-separate-bulbs-contain-ideal-gases-a-and-b, KAMPION
WebVIDEO ANSWER: Hello students in this question we have to separate bulbs contains gases A. And B. And we have given that density of gas A. Is twice of density of gas B. And the. WebTwo separate bulbs contain ideal gases A and B The density of gas A is twice that of gas B The molecular mass of A is half that of gas B The two gases are a Grade Two separate. WebTwo separate bulbs contain ideal gases \( A \) and \( B \). The density of gas \( A \) is twice that of gas \( B \). The molecular mass of \( A \) is half th... WebTranscribed image text: Two separate bulbs contain ideal gases A and B respectively. The density of gas A is twice of that of the density of gas B and the molecular weight of gas A. WebQuestion: I wo separate bulbs contain ideal gases A and B, respectively. The density of gas A (da) is twice that of gas B (ds), and the molecular weight of gas A (MA) is half of that of. WebHint: We are given two separate bulbs which have ideal gases namely A and B. We have to find the ratio of pressure exerted by the gas when the density of one gas is twice of the. WebHere you can find the meaning of Two separate bulbs contain ideal gases A and B. The density of gas A is twice that of gas B. The molecular mass of A is half that of gas B. The. WebTwo separate bulbs contain ideal gases A and B. The density of A is twice as that of gas B. The molecular mass of gas A is half as that B. If two gases are at same temperature,.
Currently - Two separate bulbs contain ideal gas `A` and `B`. The density of a gas `A` is twice that of a
Two separate bulbs contain ideal gases \( A \) and \( B \). The density of gas \( A \) is twice ...
More about Two Separate Bulbs Contain Ideal Gases A And B
Two separate bulbs contain ideal gas `A` and `B`. The density of a gas `A` is twice that of a gas `B`. The molecular mass of `A` is half that of gas `B`. The two gases are at the same temperature. The ratio of the pressure of `A` to that gas `B` is
Discussion The vapour density of a aseous mixture of non-reactive gases 'A' and 'B popular

New Two separate bulbs contain ideal gas `A` and `B`. The density of a gas

New The separate bulbs contain ideal gases a and b. the density of gas a is

About The density of gas `A` is twice that of B at the same temperature the

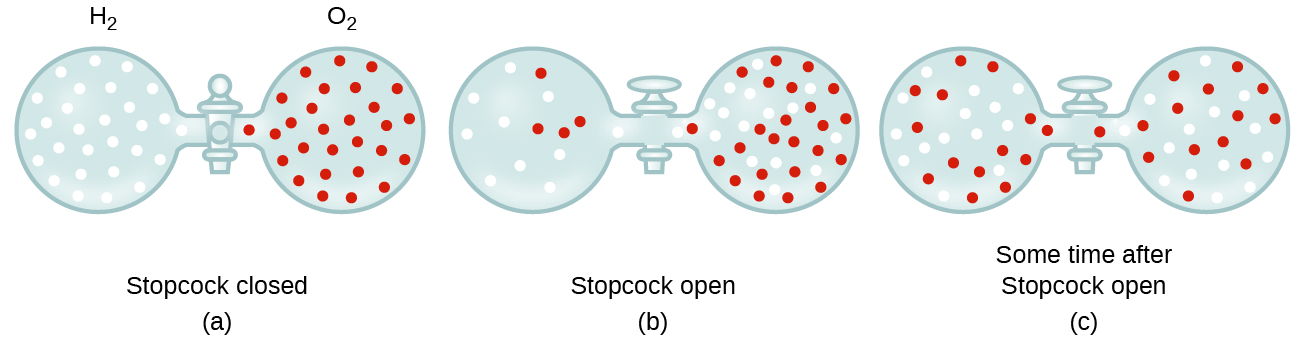
Discussion 9.4: Effusion and Diffusion of Gases - Chemistry LibreTexts update
View In the sample of soft drink, the concentration of H^+ ion is 3.8 × 10

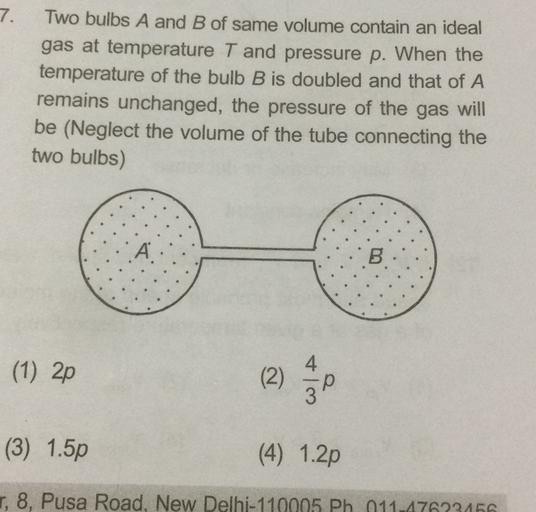
Look 7. Two bulbs A and B of same volume contain an ideal... - Physics update
Currently - Dry ice is solid carbon dioxide. A 0.05 g sample of dry ice is placed more

About Two separate bulbs contain ideal gases A and B. The density of gas is

An electron tube was sealed off during manufacture at a pressure of 1.2 New


Belum ada tanggapan untuk "Must See Two Separate Bulbs Contain Ideal Gases A And B Latest"
Posting Komentar