
WebA mixture of 2 moles of helium gas (atomic mass = 4 u), and 1 mole of argon gas (atomic mass = 40 u) is kept at 300 K in a container. The ratio of their rms speeds. is. WebA mixture of 2 moles of helium gas (atomic mass = 4 u), and 1 mole of argon gas (atomic mass = 40 u) is kept at 300 K in a container. The ratio of their ‘rms’ speeds [. WebWe start by determining the number of moles of gas present. We know that 22.4 liters of a gas at STP equals one mole, so: 867 L × 1 mol 22.4 L = 38.7 mol. We also.
A Mixture Of 2 Moles Of Helium Gas, A mixture of 2 moles of helium gas `(atomic mass=4amu)` and 1 mole of argon gas, 3.07 MB, 02:14, 448, Doubtnut, 2020-07-12T08:09:42.000000Z, 19, A mixture of 2 moles of helium gas `(atomic mass=4amu)` and 1 mole of, www.youtube.com, 1280 x 720, jpeg, , 20, a-mixture-of-2-moles-of-helium-gas, KAMPION
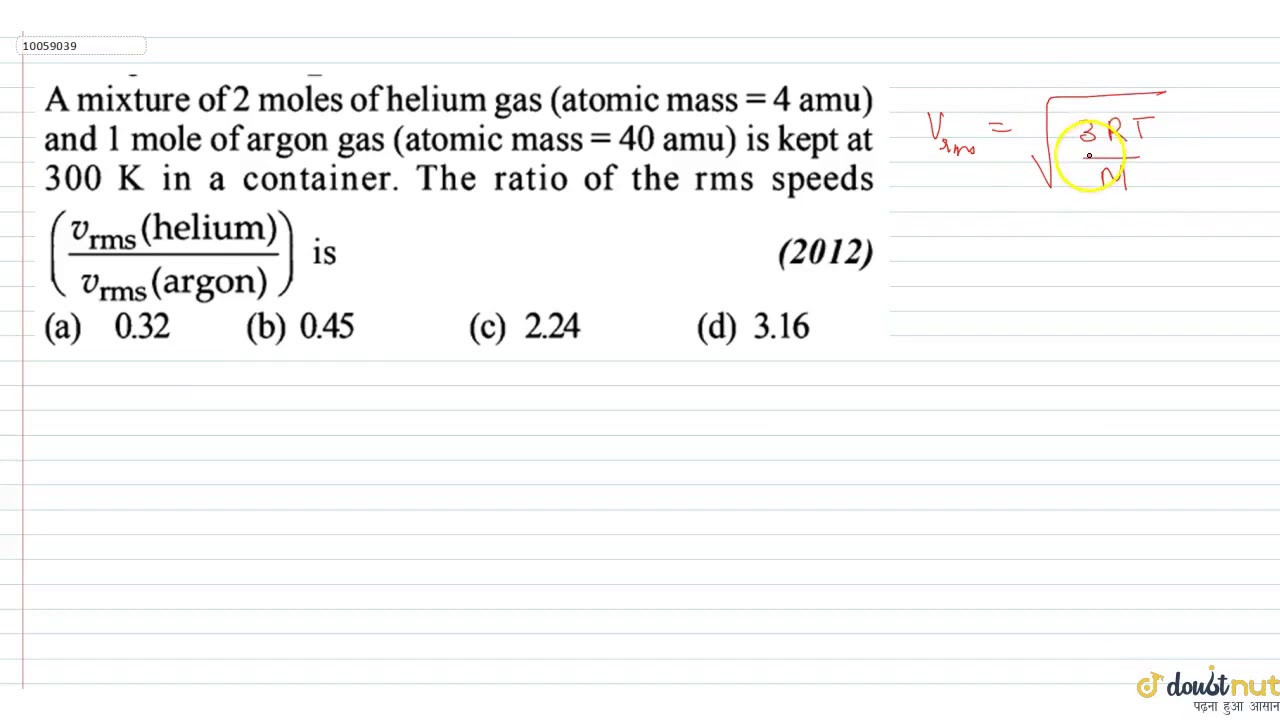
WebA mixture of 2 moles of helium gas (atomic mass =4 u), and 1 mole of argon gas (atomic mass = 40 u) is kept at 300 K in a container. The ratio of their rms speeds is close. WebNTA Abhyas 2022: A mixture of 2 moles of helium gas (atomic mass 44 ) and 1 mole of argon gas (atomic mass =404 ) is kept at 300 K in a container. The WebR = 0.083145 L ⋅ bar/mol ⋅ K. T = 273.15 K (standard temperature) We can solve for the ideal molar volume ¯¯ ¯V ideal, and then from that determine the mol s of. WebQuestion: A mixture of 2 moles of helium gas (atomic mass = 4 amu) and 1 mole of argon gas (atomic mass = 40 amu) is kept at 300 K in a container. The ratio of the rms speeds. WebA mixture of 2 moles of helium gas (atomic mass =4amu ), and 1 mole of argon gas (atomic mass = 40amu ) is kept at 300 K in a container. The ratio of the rms speeds [V rms(. WebA mixture of 2 moles of helium gas (atomic mass = 4 u ), and 1 mole of argon gas (atomic mass = 40 u ) is kept at 300 K in a container. The ratio of their rms speeds [ Vrms. WebA mixture of 2 moles of helium gas (atomic mass = 4 u), and 1 mole of argon gas (atomic mass = 40 u) is kept at 300 k in a container. The ratio of their rms speeds. WebA mixture of 2 moles of helium gas (atomic mass = 4 amu) and 1 mole of argon gas (atomic mass = 40 amu) is kept at 300 K in a container. The ratio of the rms speeds (. WebCalculation: Given: Number of moles of helium gas = 2 moles. Number of moles of argon gas = 1 moles. Atomic mass of Helium = 4. Atomic mass of Argon = 40. Molar mass of.
A mixture of 2 moles of helium gas `(atomic mass=4amu)` and 1 mole of argon gas
Articles A mixture of 2 moles of helium gas (atomic mass \( =4 \) amu), and ... trending
Other descriptions of A Mixture Of 2 Moles Of Helium Gas latest
A mixture of 2 moles of helium gas `(atomic mass=4amu)` and 1 mole of argon gas `(atomic mass=40amu)` is kept at 300K in a container. The ratio of the rms speeds `((v_(rms)(helium))/((v_(rms)(argon))` is
Currently - A mixture of 2 moles of helium gas `(atomic mass=4amu)` and 1 mole of updated

Here 21. A mixture of 2 moles of helium gas (atomic mass - Physics New
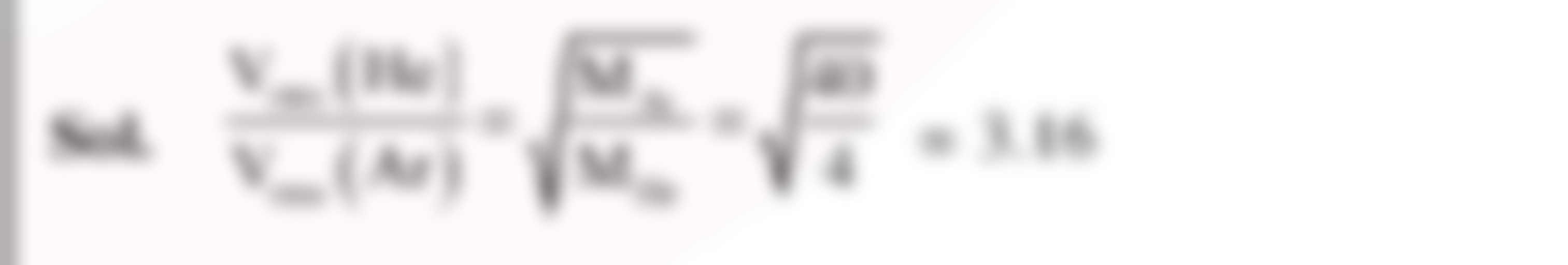
View Consider a mixture of n moles of helium gas and 2n moles of oxygen gas trending

New 4 moles of hydrogen, 2 moles of helium and 1 mole of water vapour form more

Subject Two moles of helium are mixed with n moles of hydrogen. The root mean going viral

Articles Two moles of helium are mixed with n moles of hydrogen. If CPCV = 32 Latest

Viral Two moles of helium are mixed with n moles of hydrogen. If CPCV = 32 popular

Must see PPT - Gases PowerPoint Presentation - ID:6595517 Latest
Four moles of hydrogen and 2 moles of helium form ideal gas mixture trending

Watch Two moles of helium are mixed with n moles of hydrogen. The root mean New


Belum ada tanggapan untuk "Let'S See A Mixture Of 2 Moles Of Helium Gas Viral"
Posting Komentar