
WebA sample of `HI (g)` is placed in flask at a pressure of `0.2 atm`. At equilibrium. The partial pressure of `HI (g)` is `0.04 atm`. WebQuestion From - NCERT Chemistry Class 11 Chapter 07 Question – 011 EQUILIBRIUM CBSE, RBSE, UP, MP, BIHAR BOARDQUESTION TEXT:-A sample of. Weba sample of HI is placed in a flask at a pressure of 0.2 atm at equillibrium the partial pressure of HI is 0.04 atm what is kp for given equillibrium. Question. a sample of HI is placed in a.
A Sample Of Hi Is Placed In Flask, A sample of HI (g) is placed in flask at a pressure of 0.2atm. At equilibrium the partial pressure.., 10.69 MB, 07:47, 1,720, Dr. Bandhana Sharma, 2022-01-22T14:43:44.000000Z, 19, 7.11 A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At, www.youtube.com, 1280 x 720, jpeg, , 20, a-sample-of-hi-is-placed-in-flask, KAMPION
WebA sample of HI (g) is placed in a flask at a pressure of 0.2 atm. At equilibrium, partial pressure of HI (g) is 0.04 atm. What is Kp for the given equilibrium ? 2HI (g) leftharpoons. Weba 4.369g sample of metal is placed in a flask. water is added to the flask and the total volume in the flask is read to be 126.4ml. the mass of the water, flask, and metal. WebA sample of HI(g) is placed in flask at a pressure of 0.2 atm. At equilibrium the partial pressure of HI(g) is 0.04 atm. What is Kp for the given equilibrium ?2HI (g) ⇌. WebA sample of HI(g) is placed in a flask at a pressure of 0.2 atm. At equilibrium the partial pressure of HI(g) is 0.04 atm. What is K p for the given equilibrium? 2HI(g)⇌H 2(g)+I 2(g). WebA sample Python/Flask application with Nginx proxy and a Mongo database. Open in Docker Dev Environment: NGINX / Flask / MySQL: A sample Python/Flask application with an. WebThe initial concentration of HI is 0.2 atm. At equilibrium, it has a partial pressure of 0.04 atm. Therefore, a decrease in the pressure of HI is 0.2 - 0.04 = 0.16. The given reaction is: At. WebA sample of HI(g) is placed in flask at a pressure of 0.2 atm. At equilibrium the partial pressure of HI(g) is 0.04 atm. At equilibrium the partial pressure of HI(g) is 0.04 atm.. WebA sample of HI (g) is placed in flask at a pressure of 0.2 atm. At equilibrium, the partial pressure of HI (g) is 0.04 atm. What is Kp for the given equilibrium? 2HI (g)↽−−⇀HX2. Web1-1 or Group class. Flexible Timings. Verified Tutors. Book a Free Demo. Class 11 Tuition > Learn Exercise 7 > A sample of HI (g) is placed in flask at a pressure... A.
Reviews A sample of HI (g) is placed in flask at a pressure of 0.2atm. At equilibrium the partial pressure.. going viral
A sample of `HI(g)` is placed in flask at a pressure of `0.2 atm`. At equilibrium.
Details from A Sample Of Hi Is Placed In Flask latest
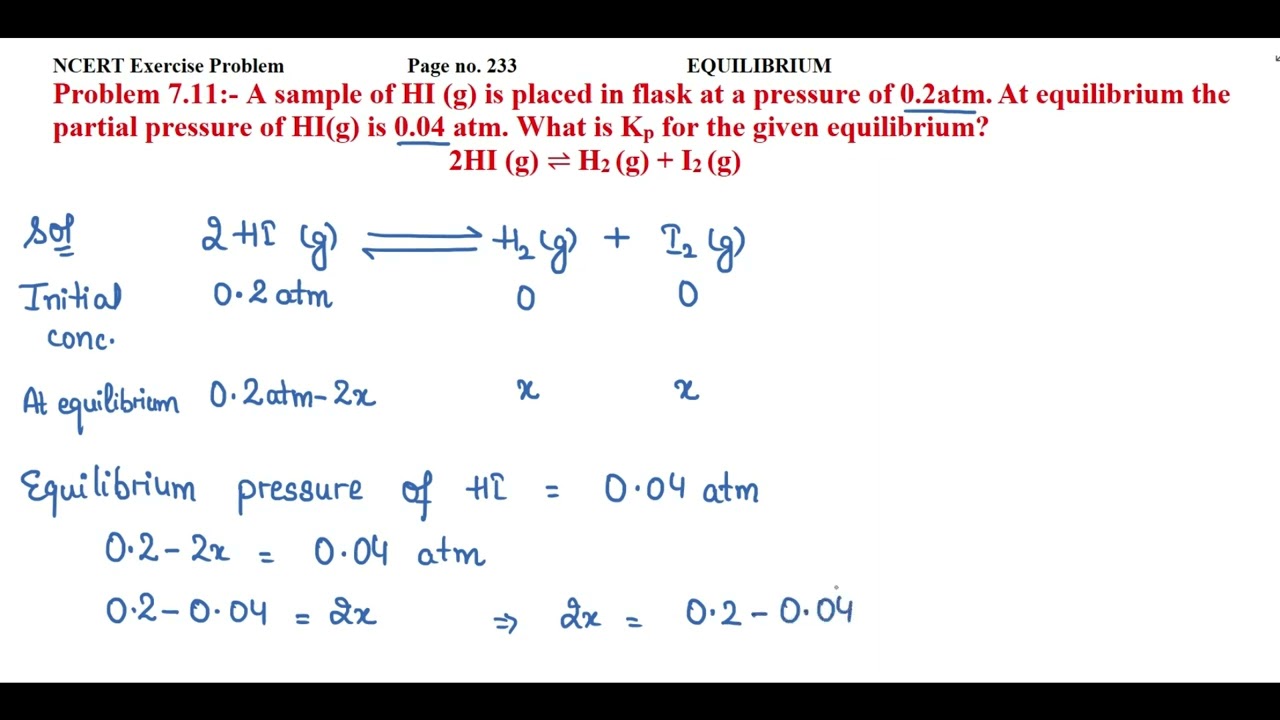
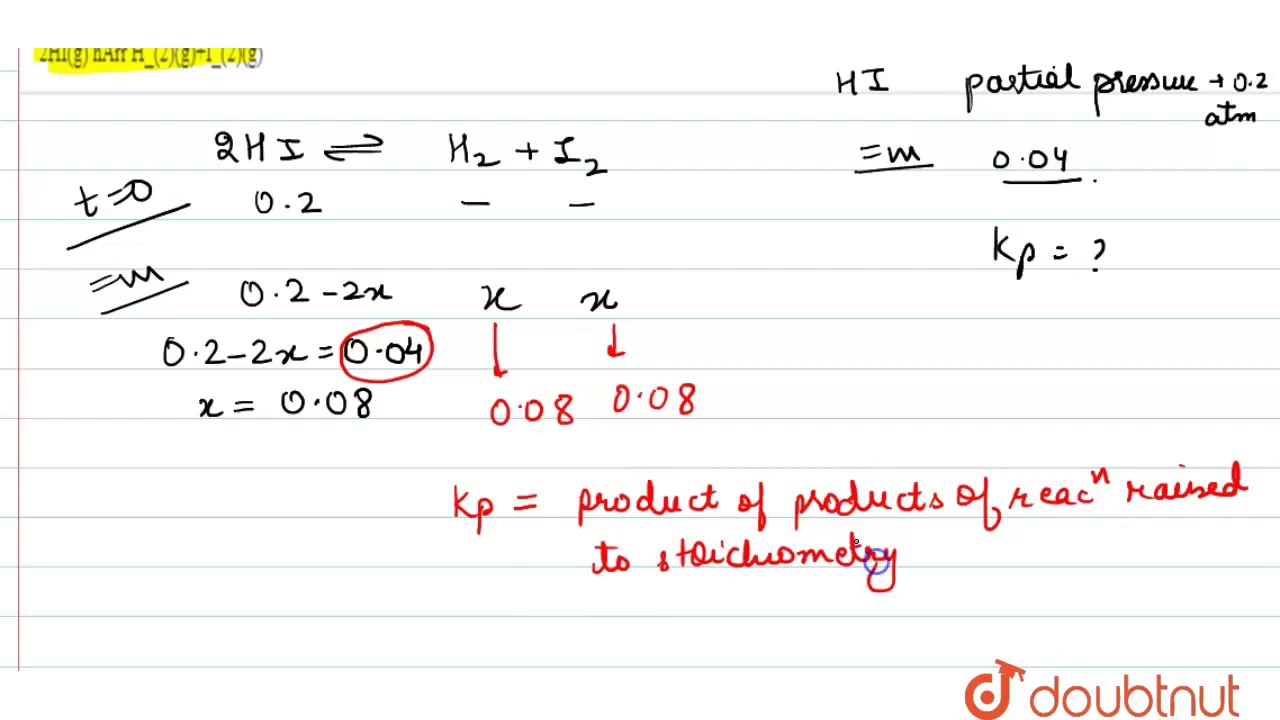
NCERT Exercise Problem Page no. 233 EQUILIBRIUM
Problem 7.11:- A sample of HI (g) is placed in flask at a pressure of 0.2atm. At equilibrium the partial pressure of HI(g) is 0.04 atm. What is Kp for the given equilibrium?
2HI (g) ⇌ H2 (g) + I2 (g)
How to find square root of non-perfect squares?
youtu.be/Ld6MOeIsdt4
How to find Log from Log table?
youtu.be/PNw2E3amEBw
How to find antilog from Antilog Table?
youtu.be/P72qe6DLqLI
How to find cube root from Log Table?
youtu.be/is_DtvpXK9s
Watch 7.11 A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At trending

Latest A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At

View A sample of HI (g) is placed in a flask at a pressure of 0.2 atm . At

Photos Top: Photo of the cylindrical sample flask placed between both HPGe

A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At updated

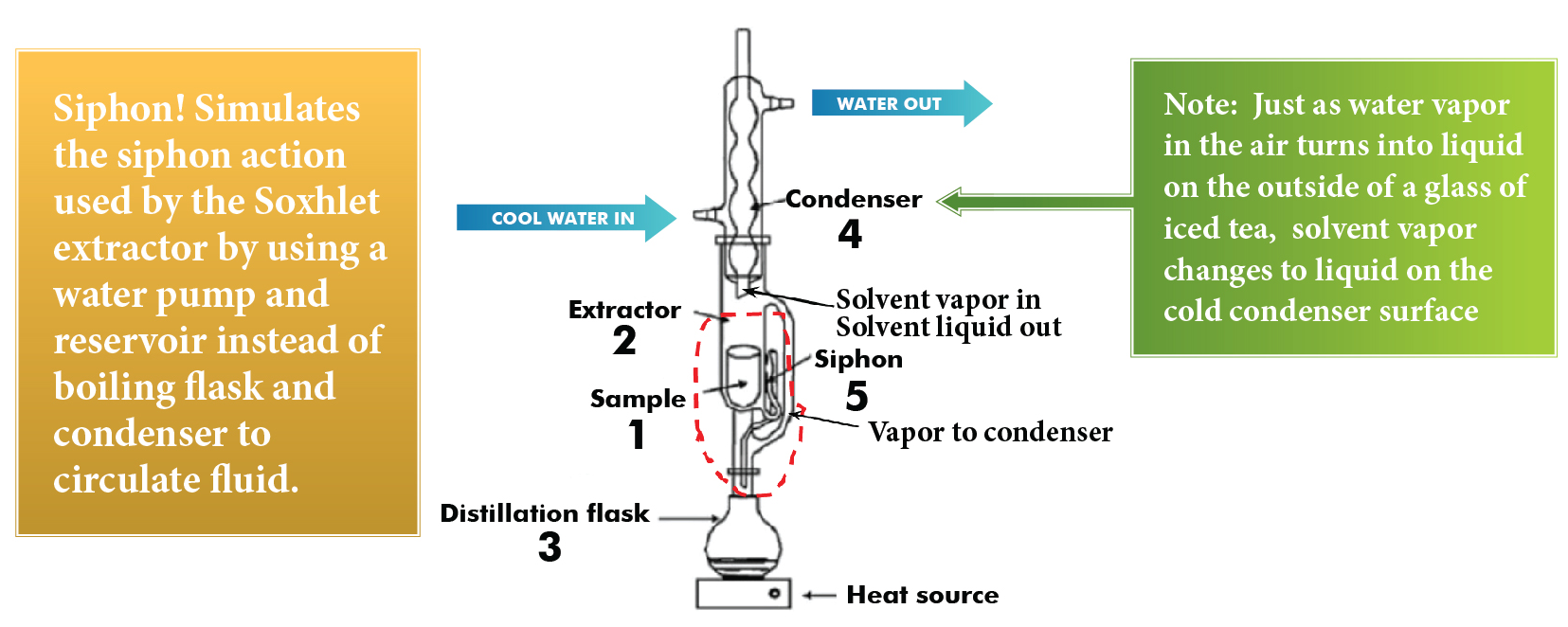
Let's see Soxhlet Extraction Siphon - Discovery Center more
Photos A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At trending

New A sample of HI (g) is placed in a flask at a pressure of 0.2 atm . At popular

Here A sample of HI (g) is placed in a flask at a pressure of 0.2 atm . At New

Viral Solved How many moles of H2 and HI will be present at | Chegg.com trending

Belum ada tanggapan untuk "Here A Sample Of Hi Is Placed In Flask Going Viral"
Posting Komentar