
WebA neon-dioxygen mixture contains 70.6 g dioxygen and 167.5g neon. If the pressure of the mixture of the gases in the cylinder is 25 bar. What is the partial pressure - Chemistry If. WebA neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If pressure of the mixture of gases in the cylinder is 25 bar. - Sarthaks eConnect | Largest. WebA neon dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If the pressure of the mixture of gases in the cylinder is 25 bar, what is the partial pressure of dioxygen and.
A Neon Dioxygen Mixture Contains 70 6, A neon-dioxygen mixture contains 70.6g dioxygen and 167.5g neon. If pressure of the mixture of gases, 12.77 MB, 09:18, 3,924, Dr. Bandhana Sharma, 2020-11-24T06:11:14.000000Z, 19, A neon - dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If, www.toppr.com, 624 x 832, jpeg, , 20, a-neon-dioxygen-mixture-contains-70-6, KAMPION
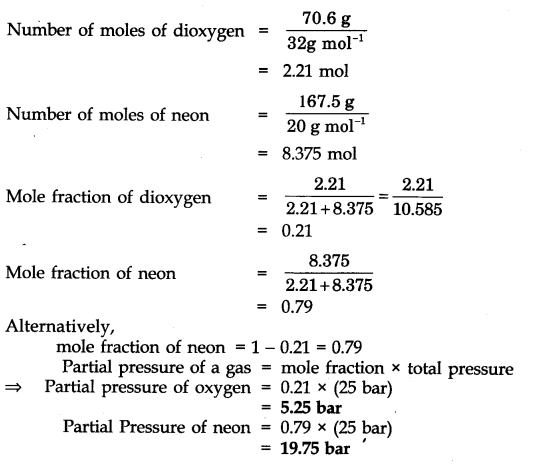
WebSolution For 11.* A neon-dioxygen mixture contains 70.6 \mathrm{~g} dioxygen and 167.5 \mathrm{~g} neon. If pressure of the mixture of 9 gases in the . The world’s only live. Web= solution : - Weight of dioxygen (O2) = 70 g Molar mass of dioxygen - 32 g mode = ". Number of moles of dioxygen 32gmat weight of neon = 16715 Molar mass of neon = 20. WebA neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If pressure of the mixture of gases in the cylinder is 25 bar. What is the partial pressure of dioxygen and. WebA neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If pressure of the mixture of gases in the cylinder is 25 bar. The partial pressure of neon is. Q. A neon. WebA neon dioxygen mixture contains 70.6 g of O 2 and I67.5 g neon. If pressure of the mixture of gases in the cylinder is 25 bar. What are the partial pressures of If pressure of the. WebQuestion From - NCERT Chemistry Class 11 Chapter 05 Question – 004 STATES OF MATTER CBSE, RBSE, UP, MP, BIHAR BOARDQUESTION TEXT:-A neon-dioxygen. WebIn a mixture of gases, each constituent gas has a partial pressure which is the theoretical pressure of that constituent gas if it alone occupied the entire volume of. WebA neon-dioxygen mixture contains 70.6 g di oxygen and 167·5 g neon. If pressure of the mixture of gases in the cylinder is 25 bar, what is the partial pressure of dioxygen and.
Must watch A neon-dioxygen mixture contains 70.6g dioxygen and 167.5g neon. If pressure of the mixture of gases
Subject A neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If pressure of the mixture of...
Details A Neon Dioxygen Mixture Contains 70 6 Next
A neon-dioxygen mixture contains 70.6g dioxygen and 167.5g neon. If pressure of the mixture of gases in the cylinder is 25bar. What is the partial pressure of dioxygen and neon in the mixture?
About A neon - dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If more

Must see A neon-dioxygen mixture contains 70.6g dioxygen and 167.5g neon.If trending

Topics A neon - dioxygen mixture contains 70.6 g a dioxygen and 167.5 g neon New

Topics If a piece of iron gains 10% of its weight due to partial rushing into Latest

New A neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. update

Look NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter (2022) New
About Mole fraction of ethanol in ethanol water mixture is 0.25. Hence Latest

Topics Mole fraction of ethanol in ethanol water mixture is 0.25. Hence trending

About A neon-dioxygen mixture contains 96g ofdioxygen and 140 g of neon. If more

Articles StudyOnline.blog for Class 11 Chemistry Chapter 5 States of Matter trending

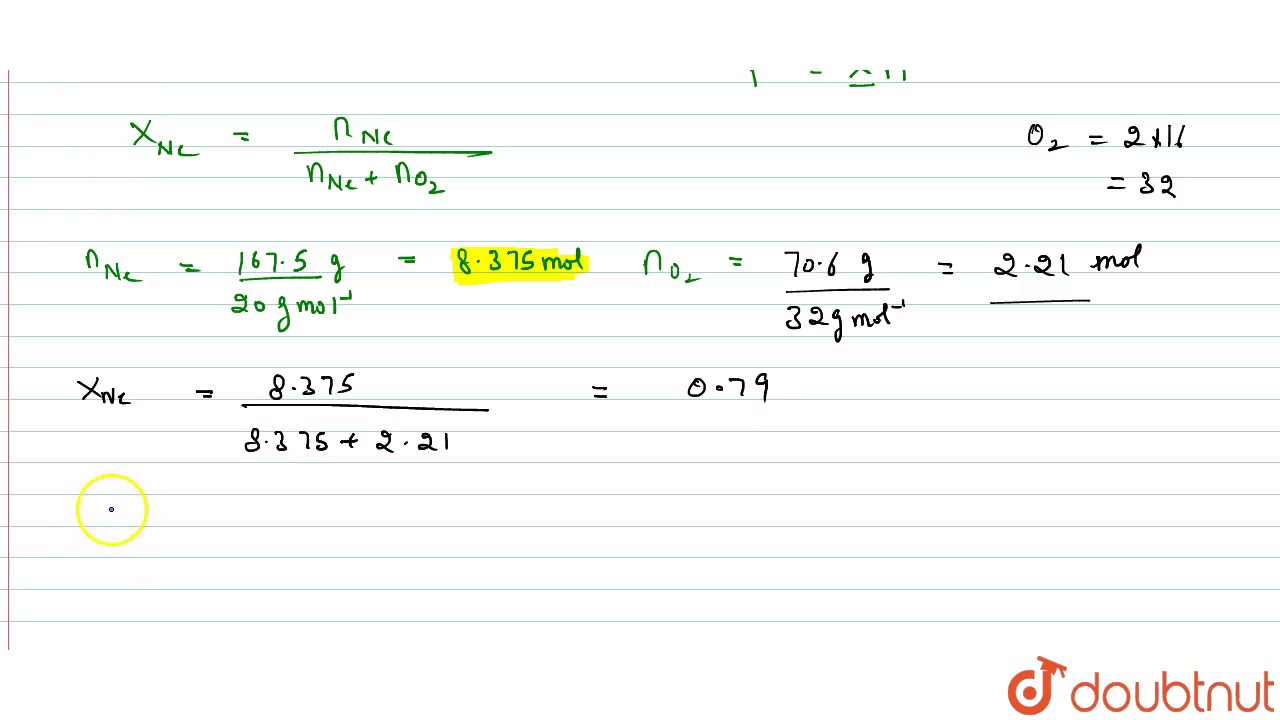
Belum ada tanggapan untuk "Topics A Neon Dioxygen Mixture Contains 70 6 Latest"
Posting Komentar