
WebThe shortest wavelength in hydrogen atoms of the Lyman series is from \[{n_1} = 1 \to {n_2} = \infty \]. The longest wavelength in the Paschen series of helium. WebFor shortest wavelength in Lyman series (i.e., series limit), the energy difference in two states showing transition should be maximum, i.e., n 2=∞. For longest wavelength in. WebThe series limit is the shortest wavelength with the fewest separated spectral lines. Lyman Series. The series was started between 1906 and 1914 by Theodore Lyman..
If The Shortest Wavelength In Lyman Series, If the shortest wavelength in Lyman series of hydrogen atom is A, then the longest wavelength in, 2.98 MB, 02:10, 2,099, Doubtnut, 2020-01-03T17:55:14.000000Z, 19, Calculate the shortest and longest wavelength in hydrogen spectrum of, brainly.in, 2100 x 2672, jpeg, lyman series wavelength shortest longest hydrogen calculate spectrum answer n1, 20, if-the-shortest-wavelength-in-lyman-series, KAMPION
WebIf the shortest wavelength in Lyman series of hydrogen atom is A, then the longest wavelength in Paschen series of H e + is : Q. If the shortest wavelength of hydrogen. WebThe shortest and the longest wavelength in Lyman series of hydrogen spectrum are:Rydberg constant, R H =109678 cm 1A. 911.7 A and 1215.7 AB. 566.4 0 A and 788.6. WebSo, the wavelength formula for Lyman series is as follows: 1 λ = R H [ 1 n 1 2 − 1 n higher 2] Shortest wavelengths in the hydrogen spectrum of Lyman series means. WebOkay, so the shortest wavelength and longest wavelength we have to determine. So for the uh for the we can calculate that the energy in the orbit and that orbit it is equal to minus. WebSolution. The wavelength of lines are given by, R H n m 1 λ = R H ( 1 n 2 - 1 m 2) For lyman series limit. n = 1. m = ∞. L R H R H ∴ 1 λ L = R H ( 1 1 2 - 1 ∞) = R H. L R H ∴ λ L. WebVIDEO ANSWER: for Lyman series. We have one over them. Dyke was our times 1/1 squared, minus one of what and spread equals R one minus one over N sway. For them.. WebWhat is the shortest wavelength in the Lyman series in \( \AA \) ?📲PW App Link - https://bit.ly/YTAI_PWAP 🌐PW Website - https://www.pw.live WebThis is the shortest wavelength of the Balmer series. or λ B = R 4. (ii) For a Lyman series, λ L 1 = R [1 2 1 − n 2 1 ]. (iii) where n = 2, 3, 4,. By putting n =.∞ in equation (iii), we. WebThe shortest and the longest wavelength in Lyman series of hydrogen spectrum are: Rydberg constant ,RH=109678 cm−1. Q. The shortest λ for the Lyman series of hydrogen.
Latest If the shortest wavelength in Lyman series of hydrogen atom is A, then the longest wavelength in New
Latest If the shortest wavelength of \( \mathrm{H} \)-atom in Lyman Series... viral
More about If The Shortest Wavelength In Lyman Series that might be interesting
If the shortest wavelength in Lyman series of hydrogen atom is A, then the longest wavelength in Paschen series of `He^(+)` is:
Latest Calculate the shortest and longest wavelength in hydrogen spectrum of New

Subject if the shortest wavelength of the spectral line of H atom in Lyman

Images The shortest wavelength in the Lyman series of hydrogen spectrum is 912

Here The shortest wavelength in hydrogen spectrum of Lyman series when R(H more

View If the shortest wavelength of Lyman series of H atom is x then the popular

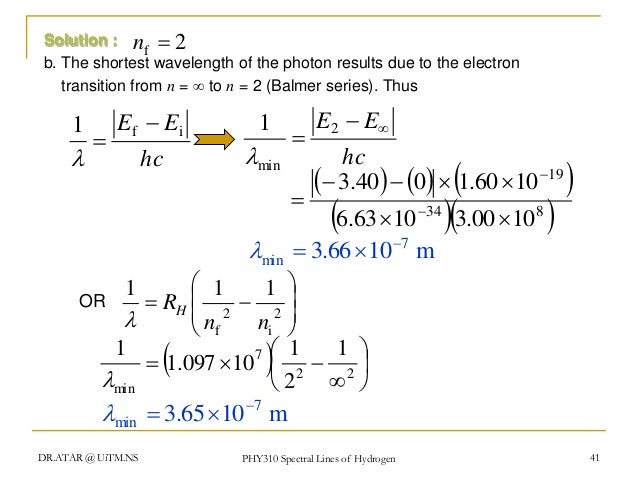
About The shortest wavelength for the Lyman series is 912A∘. Find the trending

Reviews SOLVED:The wavelengths of the Lyman series for hydrogen are given by
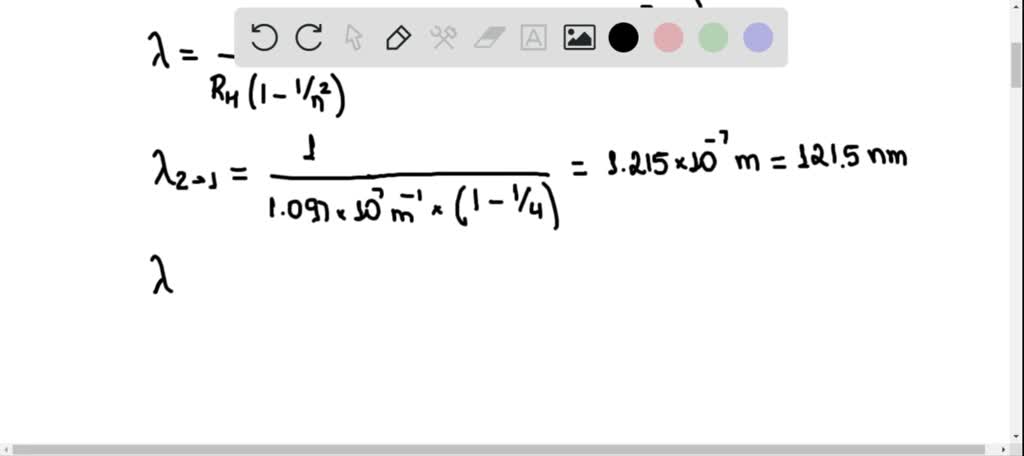
Must see Calculate the wavelength and frequency of limiting line of Lyman series Latest

About Solved The shortest wavelength of the hydrogen Lyman series | Chegg.com popular

Topics What is the Lyman series? - powerpointban.web.fc2.com update
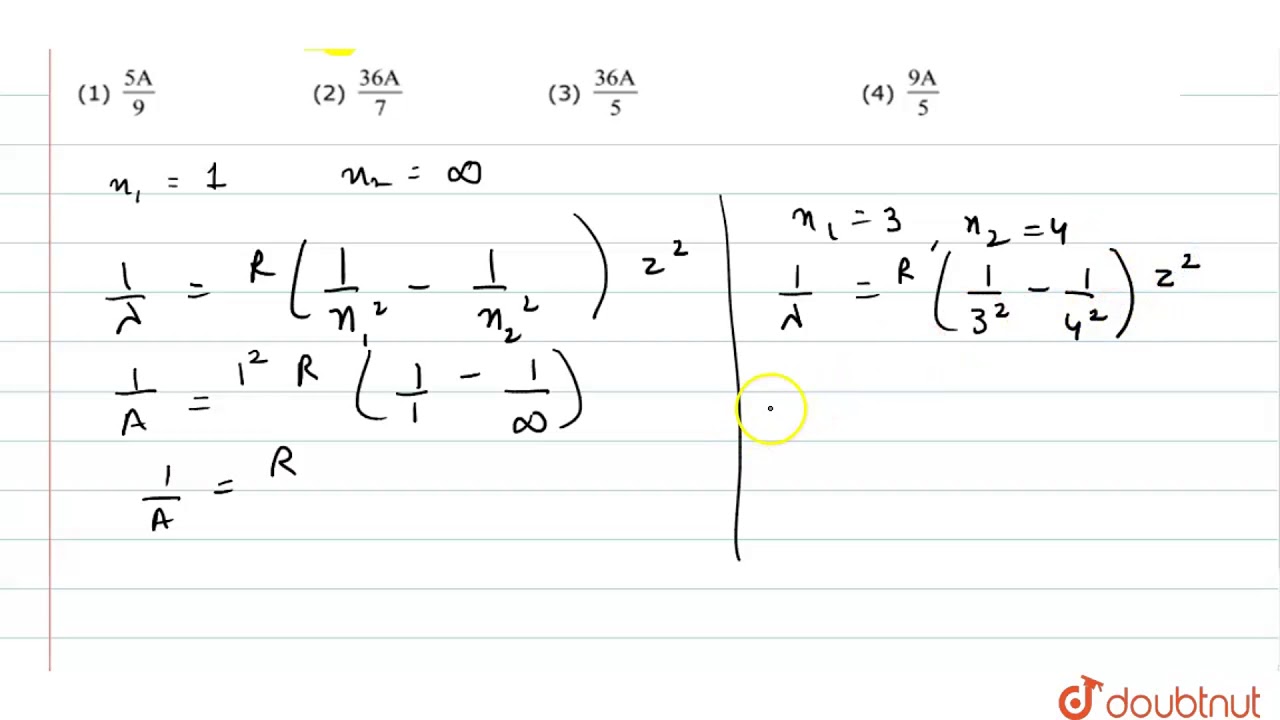

Belum ada tanggapan untuk "Images If The Shortest Wavelength In Lyman Series Article"
Posting Komentar