
WebClick here👆to get an answer to your question ️ The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm^-1 . Calculate its molar conductivity. Solve Study Textbooks Guides.. WebThe conductivity of 0.20 mol L −1 solution of KCl is 2.48 × 10 −2 S cm −1. Calculate its molar conductivity and degree of dissociation (α). Given λ 0 (K +) = 73.5 S cm 2 mol. Weba A c e t a t e i o n ( C H 3 C O O −) ^ {a} Acetate ion (CH_ { 3} COO^ { -}) a Acetate ion(C H 3. . C OO−). Therefore, the conductivity of 0.01 M (or 0.01 N) KCl solution is.
The Conductivity Of 0 20 M Solution Of Kcl, The conductivity of `0.20M` solution of `KCl` at `298K` is `0.0248 S cm^(-1)` . Calculate its mo..., 2.91 MB, 02:07, 4,044, Doubtnut, 2019-09-24T08:03:19.000000Z, 19, The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm^(-1, www.doubtnut.com, 1200 x 677, png, , 20, the-conductivity-of-0-20-m-solution-of-kcl, KAMPION
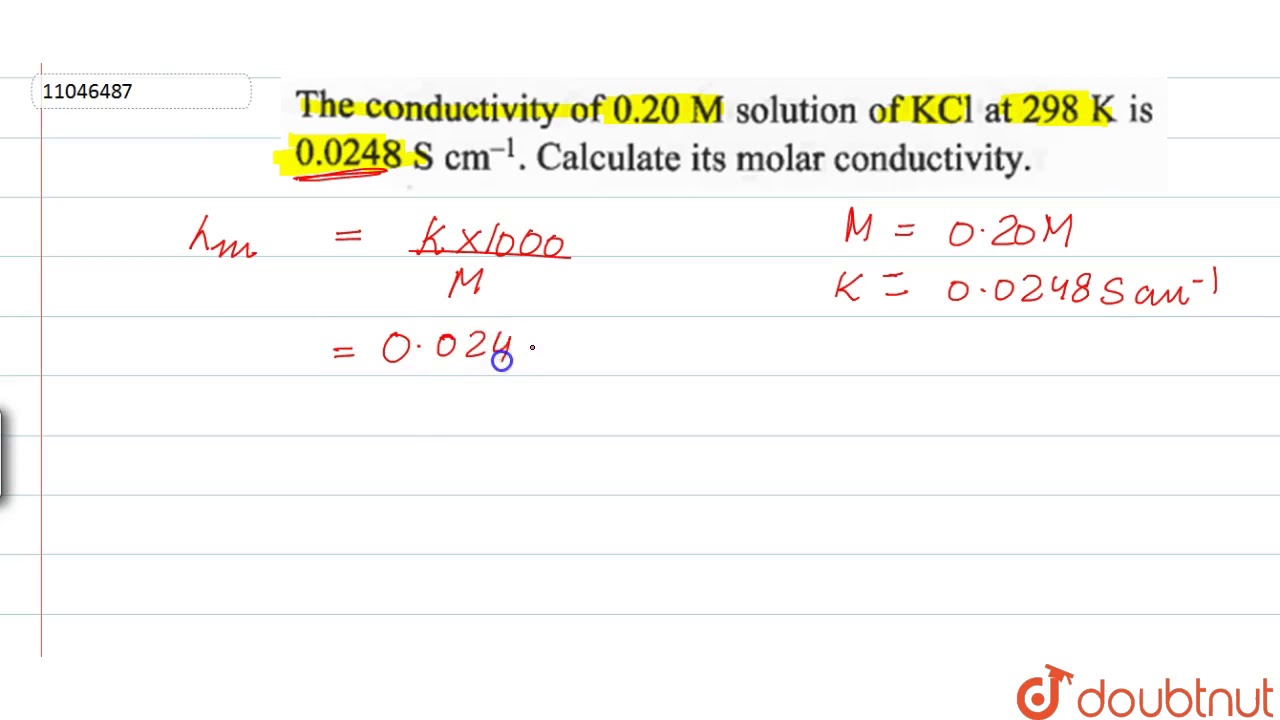
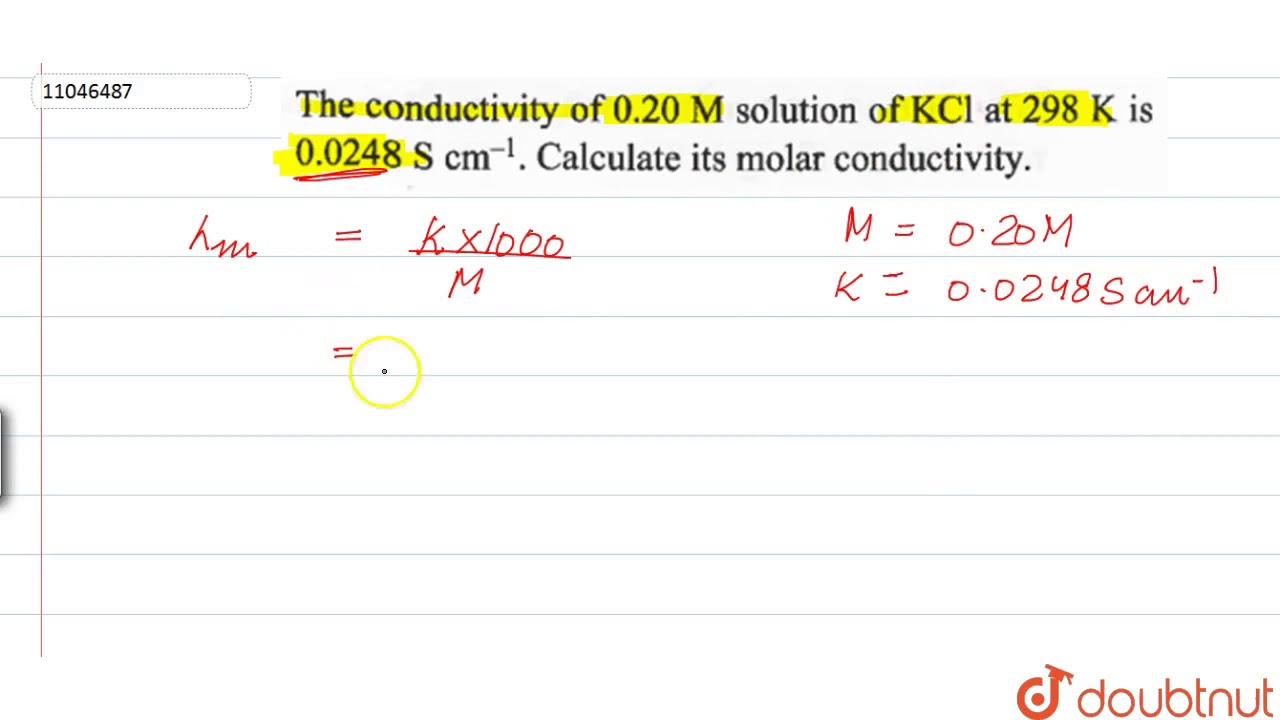
WebThe conductivity of 0.20 M solution of K C l at 298 K is 0.0248 S c m − 1. Calculate its molar conductivity. Calculate its molar conductivity. Q. Electrolytic conductivity of 0.02 mol/l. WebGive simple chemical tests to distinguish between the following pairs of compounds. (i) Propanal and Propanone. (ii) Acetophenone and Benzophenone. (iii). WebAnswer of The conductivity of a 0.20 M solution of KCl at 298 K. Call Us 07019-243-492; All Courses WebCalculate its molar conductivity. Chapter 3: Electrochemistry Chemistry Class 12 solutions are developed for assisting understudies with working on their score and increase. Webκ = 0.0248 S cm−1 and c = 0.20 M. Formula of molar conductivity, Λm = ( k × 1000) / M. Λm = (0.0248 × 1000) / 0.2. Λm = 124 S cm2 mol−1. Molar conductivity could b. Webt, 0 C 0,01 M: 0,02 M: 0,1 M: 1,0 M: Om-1 m-1: 0 : 0,0776 : 0,1521 : 0,715 : 6,541 : 2 : 0,0824 : 0,1612 : 0,757 : 6,886 : 4 : 0,0872 : 0,1705 : 0,800 : 7,237 : 6 : 0 ... WebPotassium Chloride (KCl) dissolves readily in water. A 1 M stock solution can be prepared by dissolving 74.55 g KCL (Molecular Weight: 74.55) in water to a final volume of 1L. In the.
News The conductivity of `0.20M` solution of `KCl` at `298K` is `0.0248 S cm^(-1)` . Calculate its mo... going viral
The conductivity of `0.20M` solution of `KCl` at `298K` is `0.0248 S cm^(-1)` . Calculate its molar viral
Explanation of The Conductivity Of 0 20 M Solution Of Kcl Next
Question From - NCERT Chemistry Class 12 Chapter 03 Question – 023 ELECTROCHEMISTRY CBSE, RBSE, UP, MP, BIHAR BOARD
QUESTION TEXT:-
The conductivity of `0.20M` solution of `KCl` at `298K` is `0.0248 S cm^(-1)` . Calculate its molar conductivity.
Doubtnut के साथ १००% मार्क्स पायें, आज ही डाउनलोड करें :-
doubtnut.app.link/91GzfmKxjP
OR ask your doubts on Whatsapp No --- 8400400400
Chemistry Solutions for Class 9, class 10, class 11, class 12 and IIT-JEE aspirants
Doubtnut provides Chemistry Solutions for Class 9, class 10, class 11, class 12 and IIT-JEE aspirants. The solutions are arranged chapter-wise and within each chapter they are arranged by topic-wise.
You can start learning with chapter-wise video tutorials of Chemistry books including NCERT Textbook of Chemistry, Cengage Chemistry, P Bahadur, A2Z Chemistry, Narendra Awasthi, MS Chouhan, Himanshu Pandey, Dinesh Companion Chemistry…
Physics: doubtnut.com/physics
Chemistry: doubtnut.com/chemistry
Biology: doubtnut.com/biology
Score 100% With Doubtnut
Prepare your class 12 Chemistry with our intuitive video solutions and score well in your final academic exam 2019-20.
Students can find instant video solutions on our renowned doubt solving app or even on our website by clicking the doubt question from their textbook of class 12 maths, physics, chemistry, biology.
The video tutorials are helpful in preparation for the entrance exams like IIT JEE Main and Advanced, and NEET exam. Students can resolve their doubts with self-explanatory video solutions of questions from popular books of Maths, Chemistry, and Biology are solved for easy and swift learning.
Student can prepare for IIT JEE at doubtnut.com/iit-solutions
Student can prepare for NEET at doubtnut.com/exams/neet
Doubtnut app provides free online video solutions that help students to understand each topic and retain what they have learnt for a longer duration. It further helps in creating a stronger foundation for future classes.
Subscribe to our YouTube channel to receive notifications of live classes and latest videos on various topics from subjects like – Maths, Physics, Chemistry, Biology etc. for Board exams & higher studies.
Doubtnut ऍप पर आप कक्षा ६ से १२ (IIT JEE तक) के NCERT के गणित/
भौतिक विज्ञान/रसायन विज्ञान/जीव विज्ञान/ के सभी सवालों के वीडियो देखिये और मुफ्त में खोजिये सवाल
का जवाब - 5 लाख से भी ज़्यादा सवालों के जवाब हैं Doubtnut ऍप पर
Doubtnut App and website has video solutions of all the NCERT questions from Class 6 to 12. You can download the NCERT solutions in pdf format as well.
You can also ask any question related to Math, Physics, Chemistry, Biology and get a video solution for FREE from a library of more than 5 Lakh Videos
Follow Us On Facebook
facebook.com/doubtnut
Articles The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm^(-1 trending

View The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm⁻¹ Latest

Images The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm–1

News Resistance of a conductivity cell filled with 0.02 M KCl solution is trending

News (a) Limiting molar conductivity values of KCl solution 20 extrapolated trending

Here ELECTROCHEMISTRY_ CONDUCTANCE ~ CHEMISTRY updated
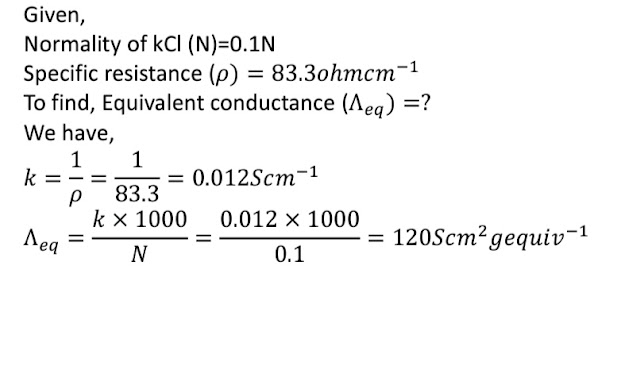
Here The resistance of a conductivity cell containing 0.001M KCl solution at update

Viral The conductivity of 0.20 M solution of KC at 298 K is 0.0248 cm trending

Reviews The conductivity of 0.20 M solution of KCI at 298 K is 0.0248 S cm
Currently - The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm^-1 update

Belum ada tanggapan untuk "Articles The Conductivity Of 0 20 M Solution Of Kcl Going Viral"
Posting Komentar