
WebA half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. [1] Often, the concept of half. WebEach of the electrodes of a galvanic cell is known as a half cell. In a battery, the two half cells form an oxidizing-reducing couple. When two half cells are connected via an electric.
For A Half Cell Reaction 1 2cl2, Oxidation-Reduction Reactions, 14.08 MB, 10:15, 60,136, Chuck Wight, 2009-03-07T00:07:22.000000Z, 19, For a half cell having reaction - 1/2Cl2 + e→ Cl-, if1MCI- solution is, brainly.in, 1184 x 1518, jpeg, , 20, for-a-half-cell-reaction-1-2cl2, KAMPION
Web/ask/question/for-a-half-cell-having-reactiondfrac12cl2-e-rightarrow-cl-if-solution-is-diluted-100/ WebHalf-Cell Definition in Chemistry. Daniell cell electrochemistry may be written as two half-reactions. A half-cell is half of an electrolytic or voltaic cell, where either. Webat 1 atm, 25 and 1 M concentration. 2. All half-cell reactions are written as reductions. 3. Magnitude of the half-cell potential is a measure of the tendency to proceed from left to. WebBalanced half-reactions are well tabulated in handbooks and on the web in a 'Tables of standard electrode potentials'. These tables, by convention, contain the half-cell. WebStudy this chemical reaction: Cr+2Cl2 → CrCl4 Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction. oxidation: ロ→口 ē. WebExplanation: Let's consider the following redox reaction. Pb + 2 Cl₂ ⇄ PbCl₄. We can identify both half-reactions. Oxidation: Pb → Pb⁴⁺ + 4 e⁻. Reduction: 2 Cl₂ + 4 e⁻. WebCorrosion is considered an electrochemical reaction resulting from the two half-cell reactions of oxidation and reduction (redox). The corrosion process occurs as a. WebIf the S.H.E. is one of the half-cells, the corresponding Nernst equation can be viewed as a description of the other half-cell. Using the cell in which the silver–silver. WebStudy this chemical reaction: Pb + 2Cl2 = PbCl4 Then write balanced half-reactions describing the oxidation and reduction that happen in this reaction. (Detailed) Question:.
News Oxidation-Reduction Reactions trending
Watch 4.2.4 (2) - 1 - Oxidation-Reduction Reactions (Engl) - difícil de entender
For A Half Cell Reaction 1 2cl2 Next
This General Chemistry lecture introduces the subject of electrochemistry by showing how to assign formal oxidation states to atoms in molecules. Reactions that involve changes in these formal oxidation states are called redox reactions, and they are prevalent in combustion. However, some systems allow us to physically separate the oxidation and reduction half-reactions and force the electrons to travel along a wire between electrodes, thereby allowing them to perform electrical work. This is the basis of the electrochemical or voltaic cell.
For a half cell having reaction - 1/2Cl2 + e→ Cl-, if1MCI- solution is

About Half cell reaction, Overall reaction (Electrochemistry part 10 for CBSE
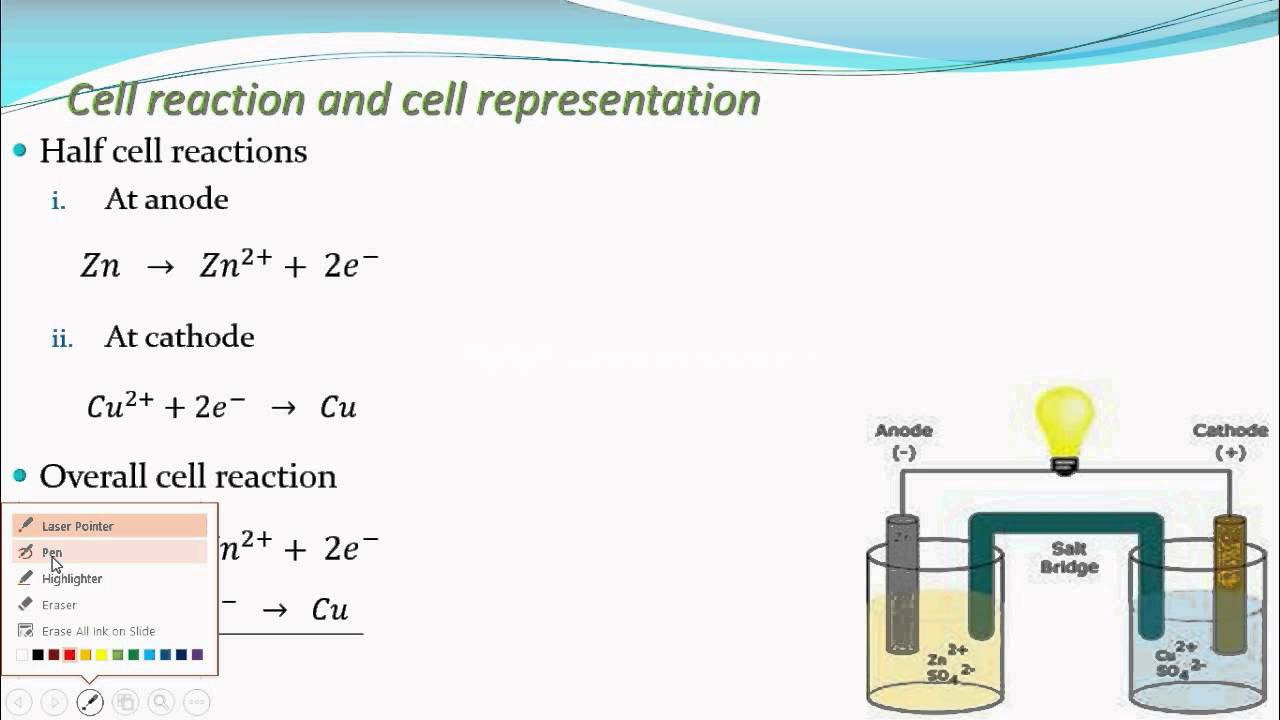
Let's see Half cell, oxidation half cell,reduction half cell(Electrochemistry trending

Solved: A Voltaic Cell Has One Half-cell With A Cu Bar In | Chegg.com more
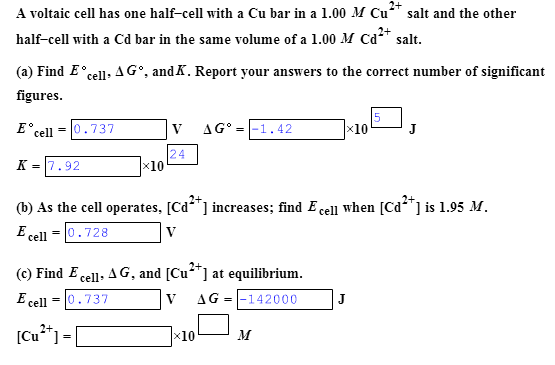
Look calculation of half cell potential electrochemistry 2 class 12

Photos Inorganic Chemistry : Electrochemistry more
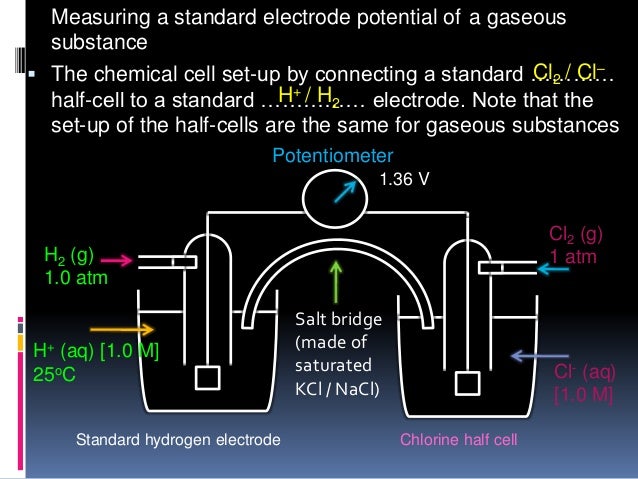
View Cells and half cells more
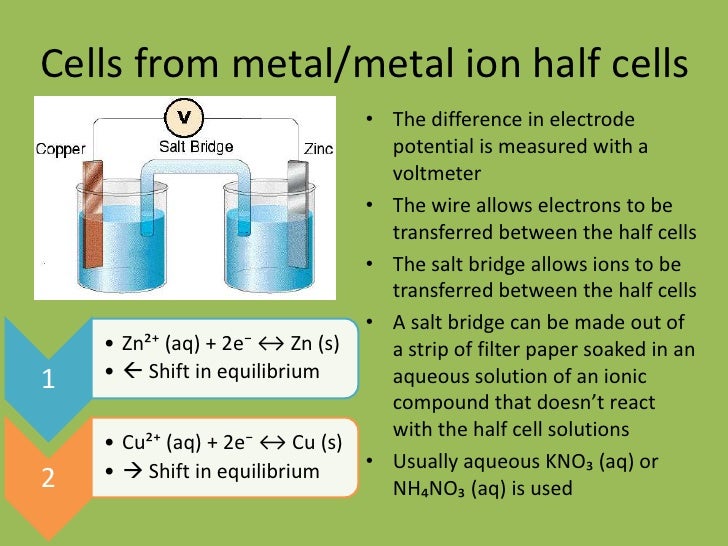
Currently - Half Cell Reaction Table viral

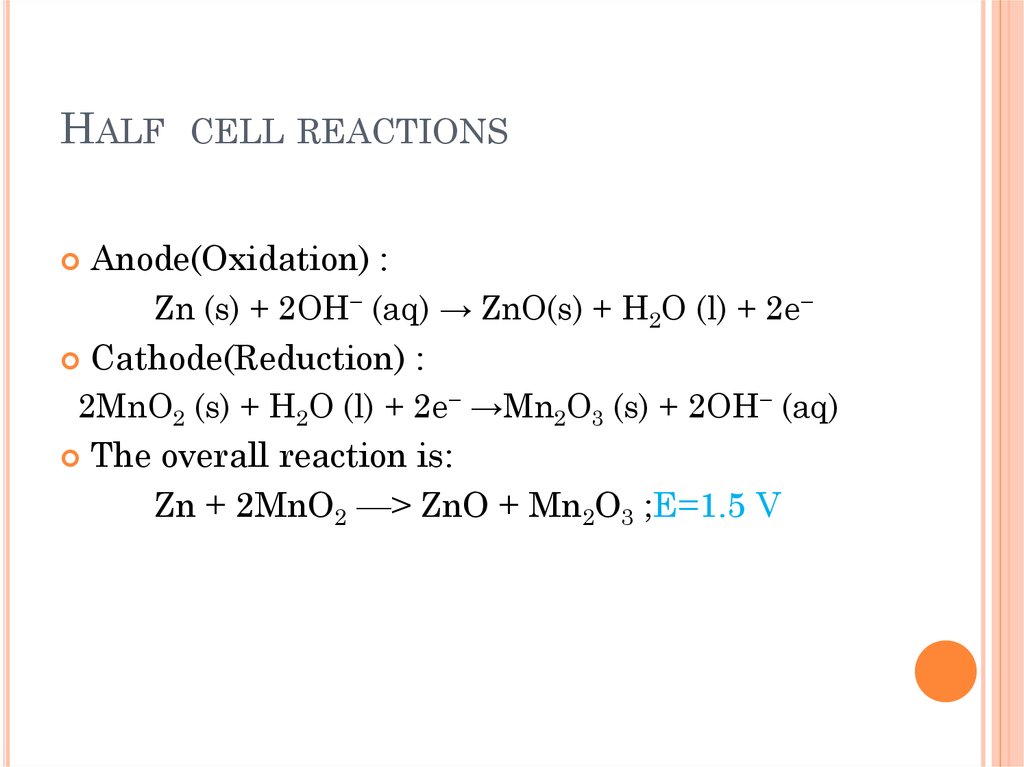
Latest Primary Batteries. The zinc-carbon cell - online presentation Latest
Reviews Solved: Complete The Two Reduction Half Reactions For The | Chegg.com



Belum ada tanggapan untuk "New For A Half Cell Reaction 1 2cl2 For You"
Posting Komentar